Premature ovarian insufficiency (POI) is a condition characterized by the loss of ovarian function before the age of 40. POI can result from one’s genetics, autoimmune diseases, or iatrogenic reasons, such as gonadotoxic chemotherapy. Several assisted reproductive technology (ART) treatments exist, such as in vitro fertilization (IVF). However, they are not without their own risks and few ovarian-sparing options exist for women requiring gonadotoxic chemotherapy. However, they are not without their own risks and few ovarian-sparing options exist for women requiring gonadotoxic chemotherapy. Therefore, alternative technologies are needed to help preserve and restore the steroidogenic and reproductive functions of the ovary.
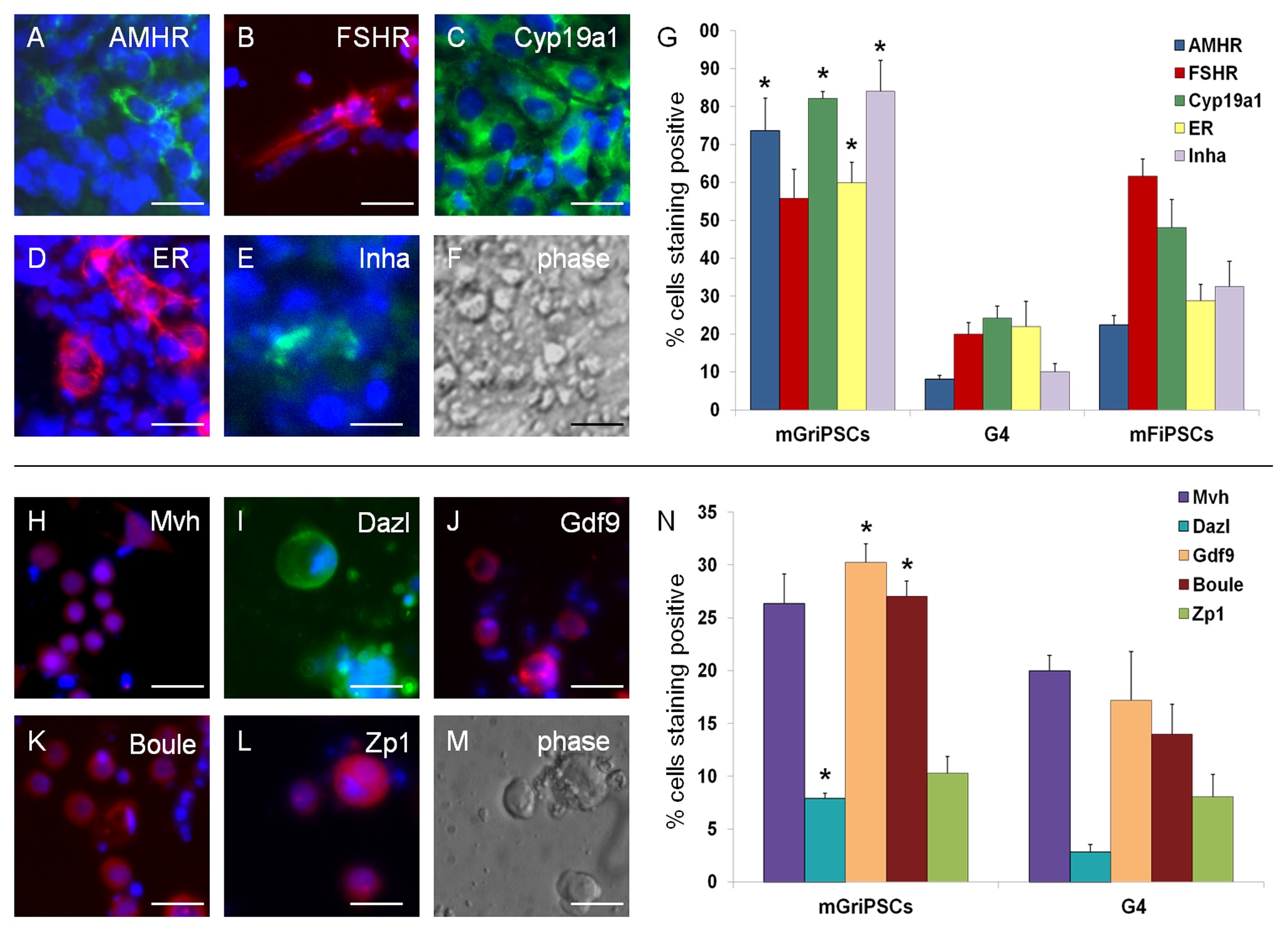
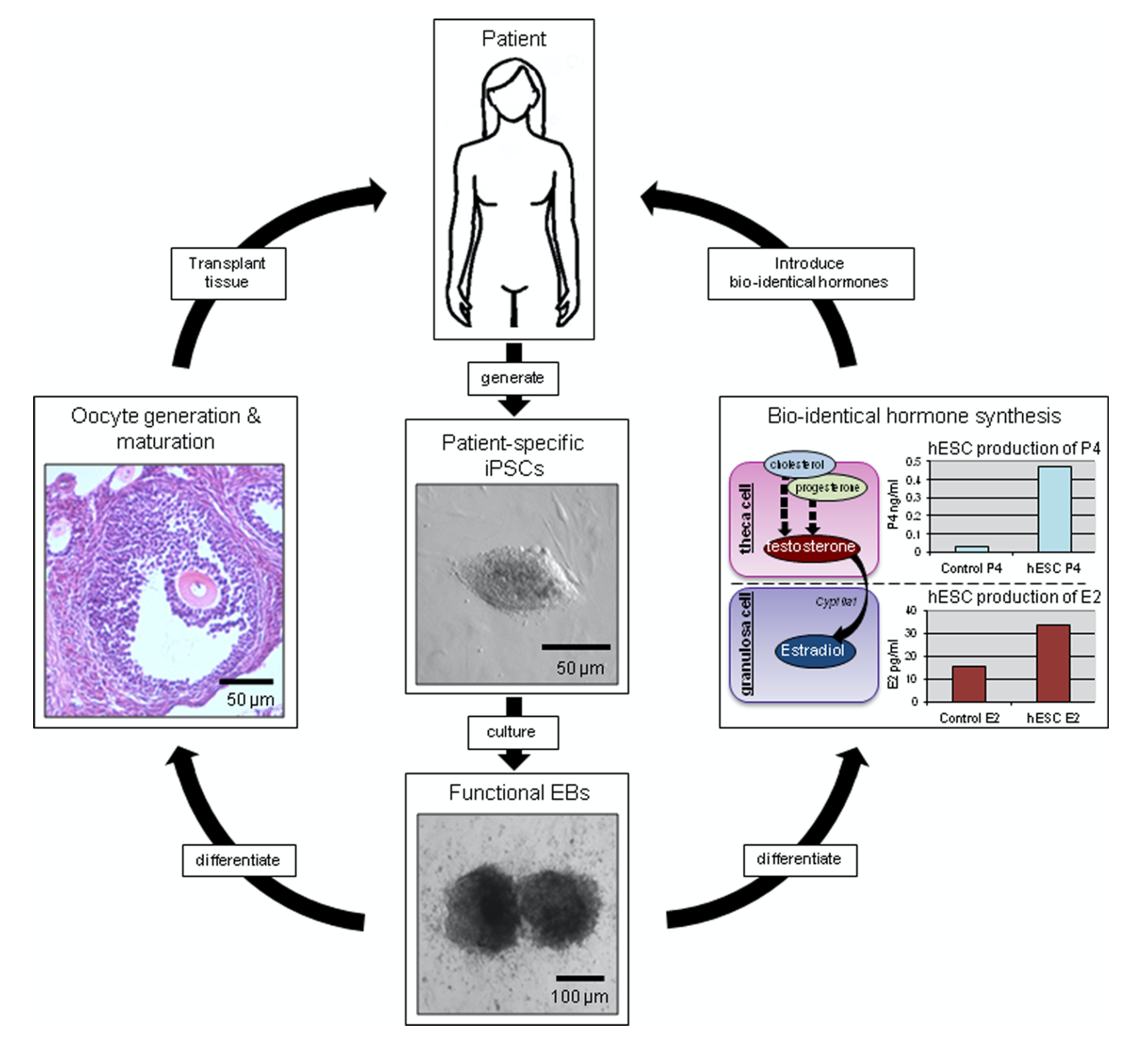
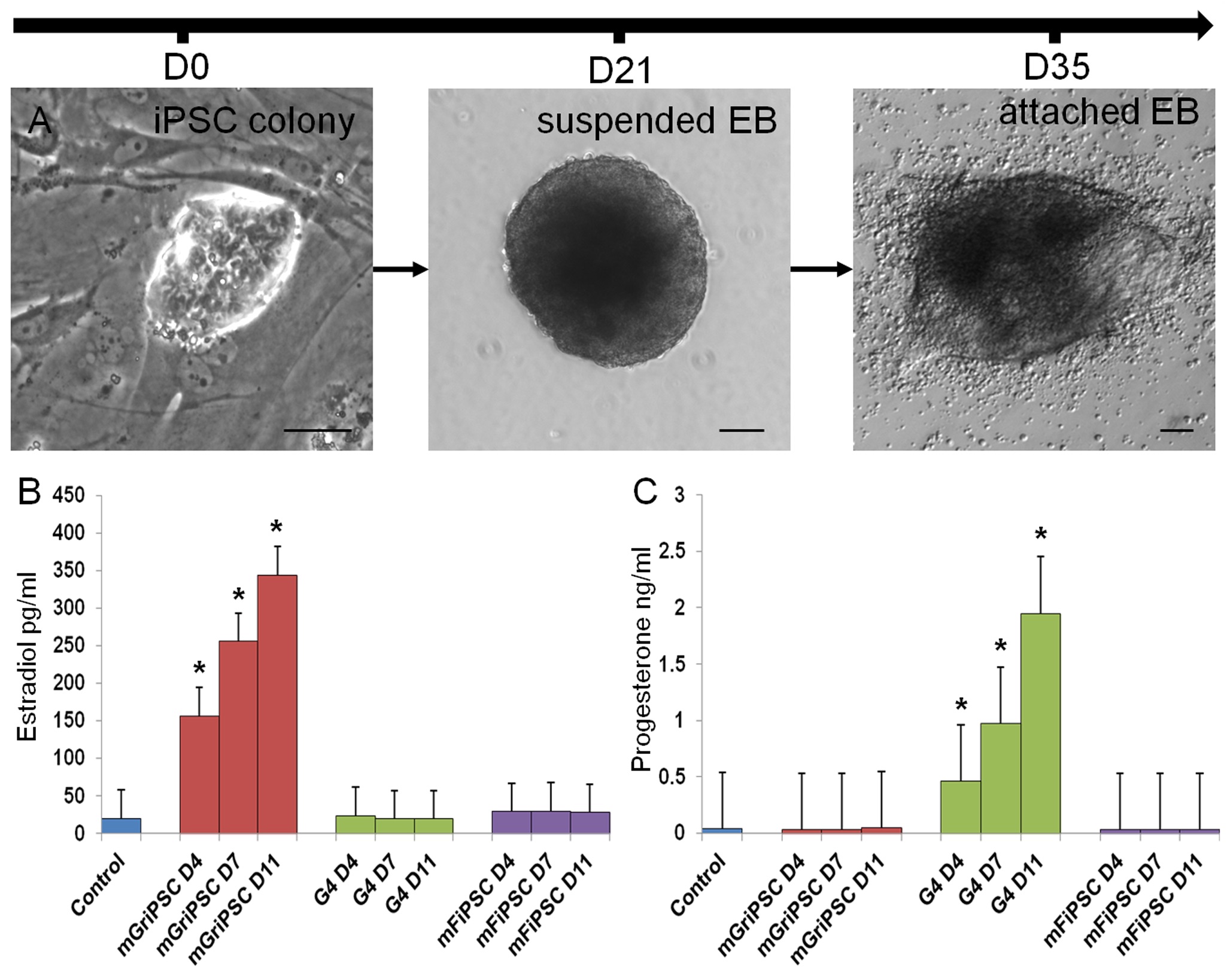
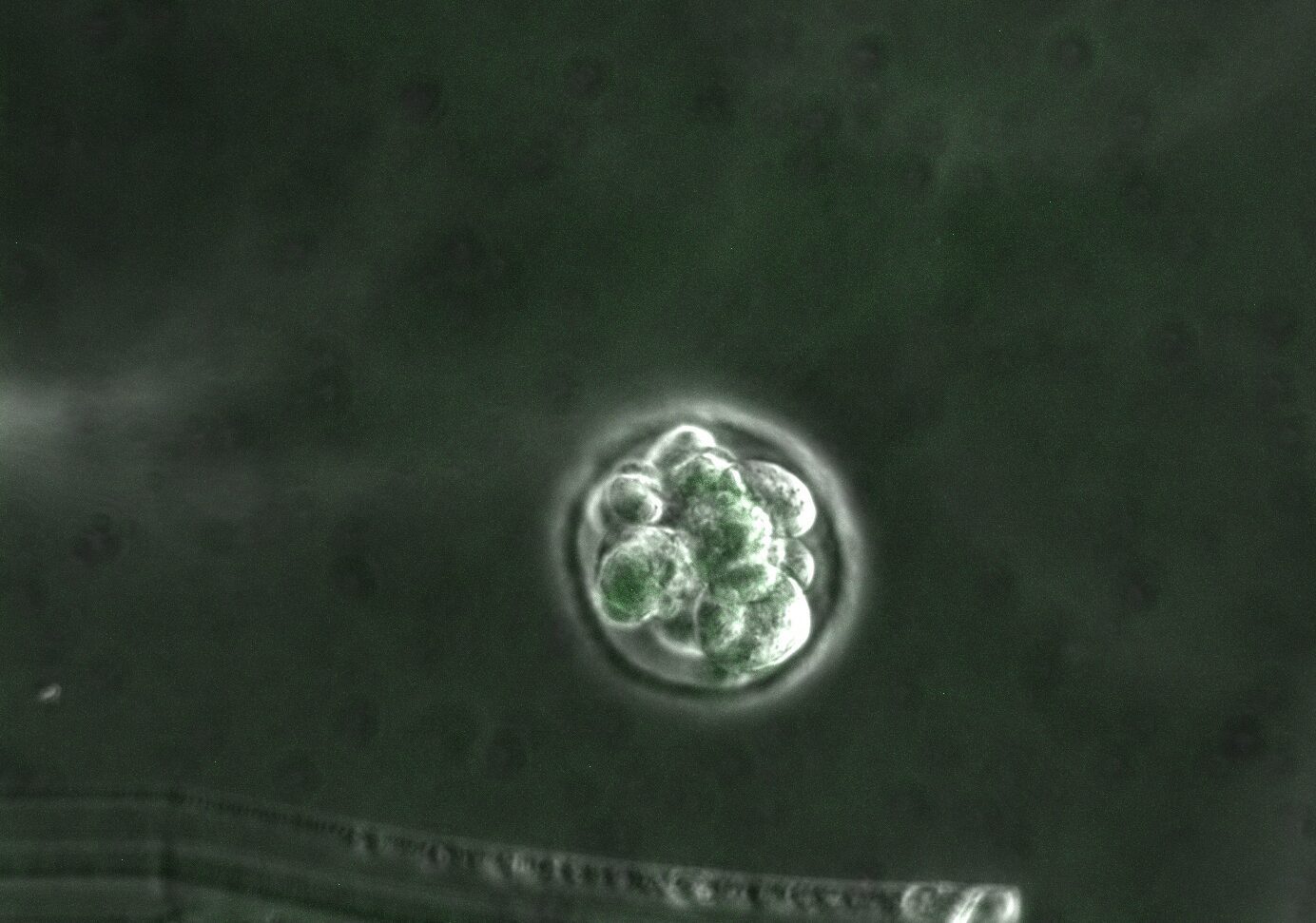
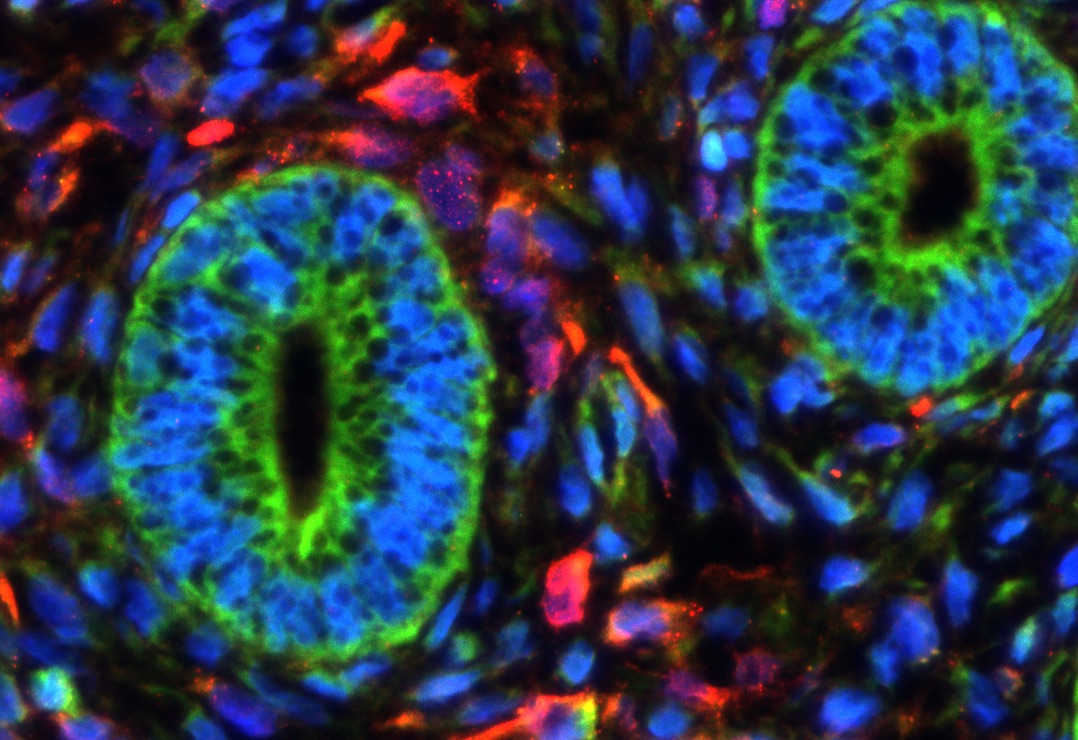
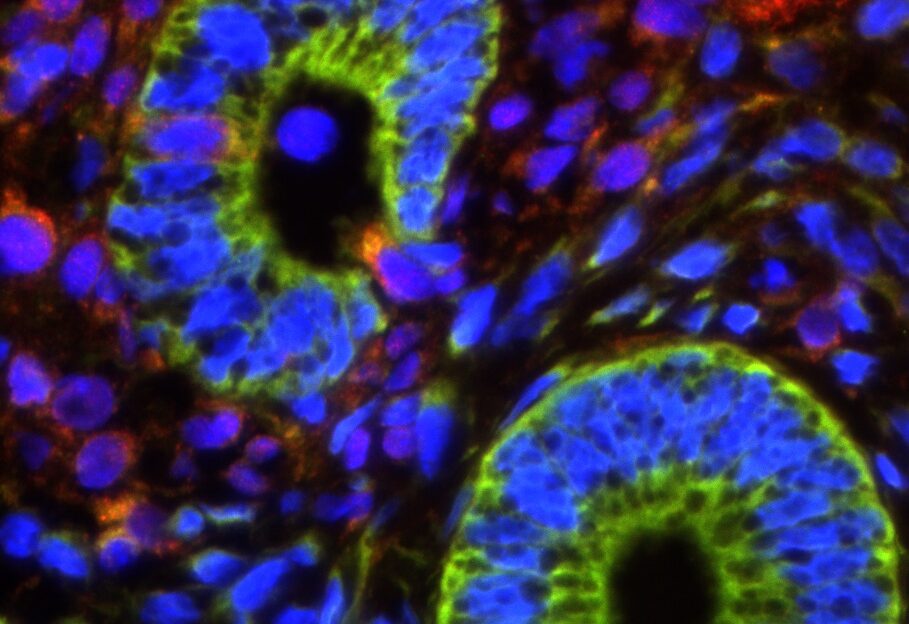
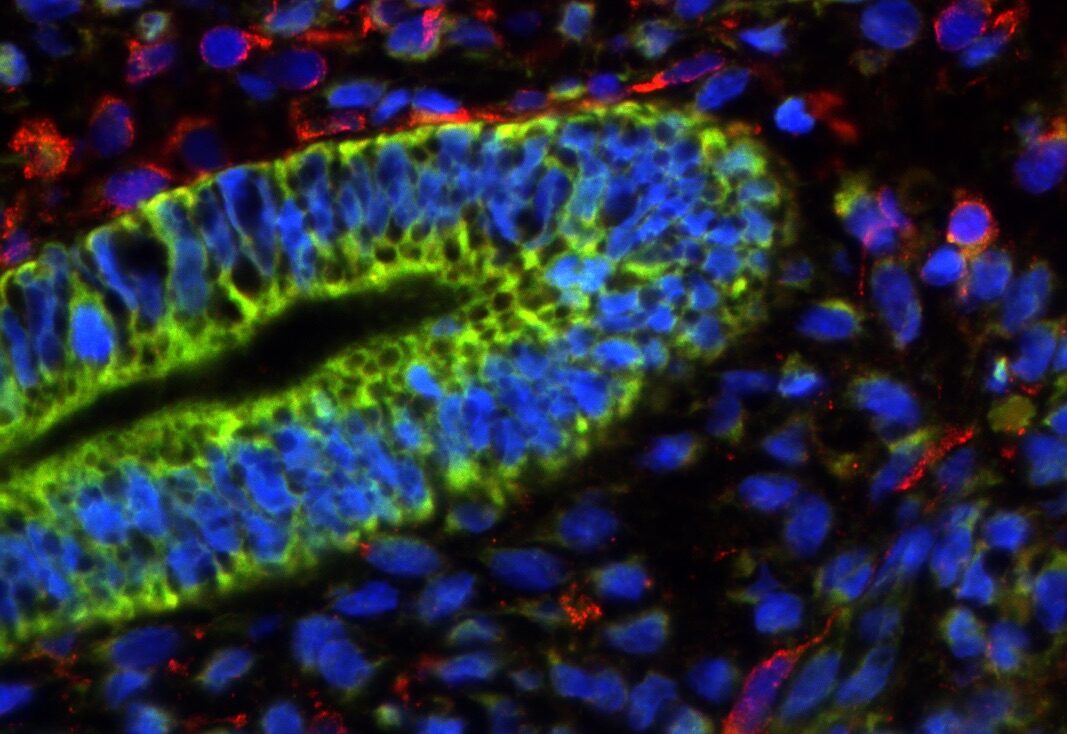
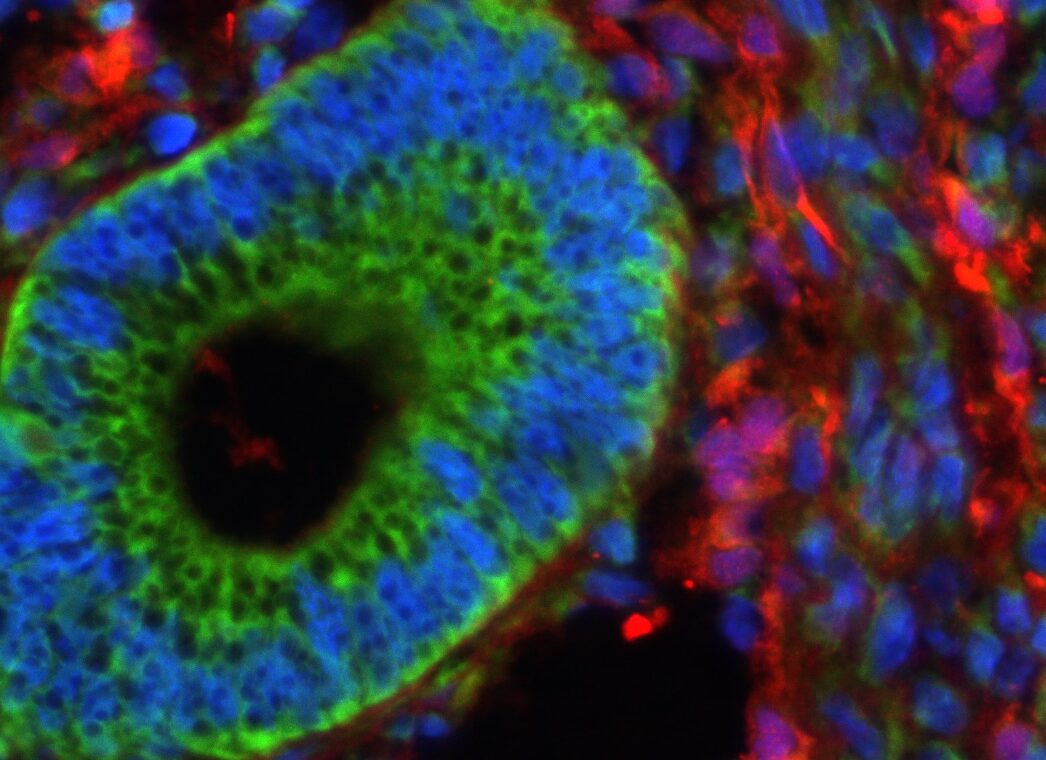
In our studies, we utilize induced pluripotent stem cells (iPSCs) to generate ovarian tissue with both steroidogenic and reproductive potential. iPSCs are likely to exhibit homotypic differentiation, which is defined as the preferential differentiation of these somatically derived iPSCs back into their cell type of origin. We therefore decided to utilize granulosa cells (GCs) for these specific studies. GCs are steroidogenic cells that surround the oocyte and are isolated and discarded from the oocyte during IVF. Not only do discarded GCs from IVF provide an accessible source of reprogrammable cells, but homotypic differentiation of GCs into ovarian-like cells elevates GCs as a promising source for iPSCs and the future of gynecological research. Ultimately, we aim to propose a model that presents the ability to generate patient-specific biomimetic hormones and autologous oocytes as therapeutic options for women with POI.
Endometriosis is a chronic inflammatory condition that occurs when tissue resembling eutopic endometrium is found outside of the uterus. Though endometriosis is a debilitating condition that occurs in about 15% of women, it is largely understudied. It can cause severe symptoms such as pain, excessive bleeding, and infertility, but its underlying biology and heterogeneous presentation remain a mystery. Interestingly, the disease burden is not associated with the severity of symptoms: while some women have large numbers of endometriosis lesions that cause little to no pain, others have few lesions that cause excruciating pain. Additionally, researchers have struggled to pinpoint the genetics that cause such variations in endometriosis. Currently, only definitive diagnosis is through surgery and pathologic confirmation, and the only available treatments include hormone therapies and surgery to remove the lesions; however, these treatments are not always effective and often lead to recurrence.
Dr. Anchan is the co-associate scientific director of basic science research at Brigham and Women’s Hospital for the Boston Center for Endometriosis, and in the Anchan Laboratory, we are actively investigating the mechanisms behind endometriosis in hopes of finding a target for therapy. In our studies, we are interested in investigating the genetics, pathophysiology, and neurogenesis relevant to the endometriosis lesions and endometriomas. Furthermore, we are also collecting questionnaires so that we can compare patient-reported outcomes to our findings to see if any correlation exists between number, size, color or location of the endometriosis lesions. Additionally, we are transforming patients’ unique endometriosis, ectopic endometrium, and endometrioma tissues into induced pluripotent stem cells, allowing us to study the development of endometriosis in a dish and further uncover its molecular mechanisms. Thus, our approach to endometriosis research comprises a broad scope of methods and a diverse set of data, enabling us to identify target genes and uncover their specific roles. We hope that these studies will one day contribute to the generation of patient-specific therapies, because treatment tailored to each patient is more effective than one-size-fits-all models.
Uterine fibroids (UF) are noncancerous growths that occur about 75% of women, with a higher incidence in African American women (about 85%). UF has multiple causes including genetic factors. Many women do not have any symptoms, but those that do may experience heavy menstrual bleeding, pain, bladder and bowel symptoms, and infertility. Our lab is part of two patient-centered clinical studies that investigate the best treatments and informed decision making for women with various symptoms and unique needs.

One of our studies is called Comparing Options for Management: Patient-Centered Results for Uterine Fibroids (COMPARE-UF). COMPARE-UF, a nation-wide study spearheaded by Duke University’s Clinical Research Institute, developed a nationwide registry of women with various demographics with UF about their symptoms and experiences before and after treatment. With over 3,000 patients enrolled, this study is forming a robust registry of patient-reported outcomes data that will fuel various studies. From this registry, we may, for example, be able to study how insurance status (public vs. private) affects the treatments that are offered and then selected by patients or how quality of life and symptoms differ from before their procedure to 6-months or 1-year after their procedure.

Our newest UF study, initiated by the Dartmouth Institute for Health Policy and Clinical Practice, is called UPFRONT. Dartmouth and its researchers have developed a decision-making tool, called the Option GridTM, to increase patient engagement in their treatment decision-making process for UF. This tool has been shown to increase patient engagement and satisfaction, in addition to helping patients weigh different treatment options and select the one that is right for them. The Option Grid also helps physicians facilitate a more thorough and efficient discussion about the right treatment options with their patients. However, it is challenging to make sure that all UF patients receive the Option Grid and that their providers find it easy to use in the clinical setting. UPFRONT seeks to evaluate the integration of Option Grid into at five clinical sites across the country, ultimately generating solutions that would allow Option Grid to be implemented at any gynecology practice.
We hope that our research in studying the neurological pathways to investigate the pain associated with endometriosis can be translated to help develop therapeutic options for other pain-related diseases as well. Additionally, Dr. Anchan has also been interested in the use of stem cells for vision research. Specifically, Dr. Anchan is interested in using induced pluripotent stem cells to generate lens cells so that we can better study various lens diseases in vitro.
Amniocyte stem cell derived cardiac tissue.